Multiple Choice
What is ΔrG°?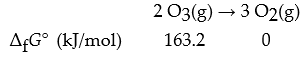
A) 326.2 kJ
B) -326.4 kJ
C) -163.2 kJ
D) 163.2 kJ
E) 54.4 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q81: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298
Q82: The maximum quantity of energy available for
Q83: Find correct statements.<br>I.A spontaneous process is a
Q84: The following reaction is exothermic.<br>2 N<sub>2</sub>O(g)→ 2
Q85: A reaction is spontaneous if:<br>I.ΔG is a
Q87: Which of the following has the highest
Q88: For the reaction<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the reaction
Q89: What is Δ<sub>r</sub>G° at 25 °C?<br>CO(g)+ 2
Q90: What is Δ<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is Δ<sub>r</sub>G°?
Q91: ?<sub>r</sub>G ? is independent of temperature.