Multiple Choice
Consider the following reaction: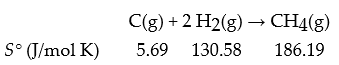
What is ΔrS° for the above reaction in J/mol K?
A) -49.9 J/mol K
B) 49.9 J/mol K
C) 80.7 J/mol K
D) 115.2 J/mol K
E) -80.7 J/mol K
Correct Answer:

Verified
Correct Answer:
Verified
Q102: Calculate the entropy change for methanol at
Q103: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q104: For the reaction SO<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ SO<sub>2</sub>Cl<sub>2</sub>(g)K<sub>eq</sub> =
Q105: Consider the reaction: AB(g)? A(g)+ B(g)<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg"
Q106: What is Δ<sub>r</sub>G° at 25 °C?<br>CO<sub>2</sub>(g)→ CO<sub>2</sub>(aq)Δ<sub>r</sub>H°
Q108: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298
Q109: The change in Gibbs energy for a
Q110: A zero ΔG means the system is
Q111: If a process is spontaneous,the reverse process
Q112: For a given reaction,Δ<sub>r</sub>H = -26.6 kJ