Multiple Choice
Consider the following reaction: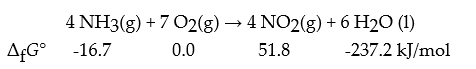
What is Keq for this reaction at 25 °C?
A) e-464
B) e464
C) 1.59
D) 0.63
E) 252
Correct Answer:

Verified
Correct Answer:
Verified
Q98: The entropy of a system always increases
Q99: A spontaneous process will occur only if
Q100: The entropy of a pure perfect crystal
Q101: The value of Δ<sub>f</sub>G at 100.0 °C
Q102: Calculate the entropy change for methanol at
Q104: For the reaction SO<sub>2</sub>(g)+ Cl<sub>2</sub>(g)→ SO<sub>2</sub>Cl<sub>2</sub>(g)K<sub>eq</sub> =
Q105: Consider the reaction: AB(g)? A(g)+ B(g)<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg"
Q106: What is Δ<sub>r</sub>G° at 25 °C?<br>CO<sub>2</sub>(g)→ CO<sub>2</sub>(aq)Δ<sub>r</sub>H°
Q107: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q108: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298