Multiple Choice
Consider the following reaction: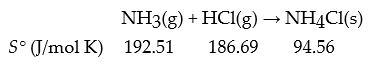
What is ΔrS° for this reaction in J/mol K?
A) -284.6 J/mol K
B) 284.6 J/mol K
C) -92.3 J/mol K
D) 94.6 J/mol K
E) 92.3 J/mol K
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q14: Standard Gibbs energy of formation requires the
Q15: Consider the reaction: AB(g)→ A(g)+ B(g)<br>(K<sub>eq</sub> =
Q16: Which of the following combinations of signs
Q17: Which of the following processes would result
Q19: As the activity a of a substances
Q20: Calculate the temperature for which K<sub>eq</sub> for
Q21: For an exothermic reaction to be non
Q22: Which of the following quantities for an
Q23: Choose the INCORRECT statement.<br>A)The third law of