Multiple Choice
Consider the following reaction: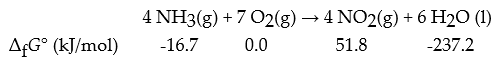
What is ΔrG° for this reaction in kJ?
A) -1282
B) -1149
C) -169
D) 169
E) 1149
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The equilibrium constant for the reaction below
Q9: The normal melting and boiling points of
Q10: The Gibbs energy change for a reaction
Q11: For the reaction PCl<sub>5</sub> (g)⇌ PCl<sub>3</sub> (g)+
Q12: Choose the INCORRECT statement about coupled reactions.<br>A)The
Q14: Standard Gibbs energy of formation requires the
Q15: Consider the reaction: AB(g)→ A(g)+ B(g)<br>(K<sub>eq</sub> =
Q16: Which of the following combinations of signs
Q17: Which of the following processes would result
Q18: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the