Multiple Choice
Consider the following reaction: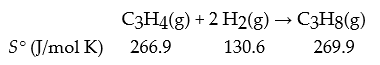
What is ΔrS° in J/mol K?
A) 127.6 J/mol K
B) -127.6 J/mol K
C) 3.0 J/mol K
D) -258.2 J/mol K
E) -3.0 J/mol K
Correct Answer:

Verified
Correct Answer:
Verified
Q44: Under which of the following conditions would
Q45: Entropy changes depend on the quantities of
Q46: For the reaction<br><br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the reaction
Q47: For the reaction N<sub>2</sub>O<sub>3</sub>(g)→ NO(g)+ NO<sub>2</sub>(g)Δ<sub>r</sub>G° =
Q48: Indicate the statement(s)which is (are)true for the
Q50: A non spontaneous reaction can be made
Q51: Consider the reaction:<br>H<sub>2</sub>X(g)→ HX(g)+ X(g)<br>Δ<sub>r</sub>H° = 18.4
Q52: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298
Q53: If ΔG is positive for a certain
Q54: For CdO(s)+ SO<sub>3</sub>(g)→ CdSO<sub>4</sub>(s),Δ<sub>r</sub>H° = -279.4 kJ/mol,and