Multiple Choice
What is ΔrG°?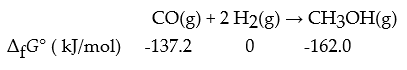
A) -24.8 kJ
B) -299.2 kJ
C) +24.8 kJ
D) 149.6 kJ
E) +299.2 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q108: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298
Q109: The change in Gibbs energy for a
Q110: A zero ΔG means the system is
Q111: If a process is spontaneous,the reverse process
Q112: For a given reaction,Δ<sub>r</sub>H = -26.6 kJ
Q114: Which material has the largest entropy?<br>A)pure water<br>B)powdered
Q115: What is Δ<sub>r</sub>G° at 25 °C?<br>N<sub>2</sub>O<sub>4</sub>(g)→ 2
Q116: For CdO(s)+ SO<sub>3</sub>(g)→ CdSO<sub>4</sub>(s),Δ<sub>r</sub>H° = -279.4 kJ/mol,and
Q117: For CO(g)+ H<sub>2</sub>(g)→ H<sub>2</sub>CO(g),Δ<sub>r</sub>H° = -5.36 kJ/mol,and
Q118: In a sealed container,the rate of dissolving