Multiple Choice
The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ.The specific heats of ice,water,and steam are 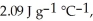
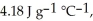 And
And 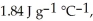 Respectively.For
Respectively.For 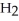 O,
O, 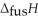 = 6.01 kJ mol-1,and
= 6.01 kJ mol-1,and 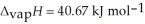 .
.
A) 12.28
B) 6.16
C) 3870
D) 7.21
E) 9.88
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Calculate the temperature for which K<sub>eq</sub> for
Q21: For an exothermic reaction to be non
Q22: Which of the following quantities for an
Q23: Choose the INCORRECT statement.<br>A)The third law of
Q24: For the reaction I<sub>2</sub>(s)+ Cl<sub>2</sub>(g)→ 2 ICl(g),Δ<sub>r</sub>H°
Q26: Entropy is related to the way in
Q27: Standard molar entropy,S<sup>∘</sup>,increases as molecular complexity increases.
Q28: Choose the INCORRECT statement.<br>A)The van't Hoff equation
Q29: If the enthalpy of vaporization of chloromethane,CH<sub>3</sub>Cl,is
Q30: For Cl<sub>2</sub>O(g)+ 3/2 O<sub>2</sub>(g)→ 2 ClO<sub>2</sub> ,Δ<sub>r</sub>H°