Multiple Choice
Choose the INCORRECT statement.
A) The van't Hoff equation is ln 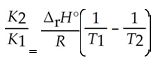
B) Keq is independent of temperature.
C) In a thermodynamic equilibrium constant expression,the activity of a gas is replaced by its partial pressure in atmosphere.
D) In a Keq expression,the activity of an aqueous species can be approximated by the numerical value of its molarity.
E) If ΔG = 0,the process is at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Choose the INCORRECT statement.<br>A)The third law of
Q24: For the reaction I<sub>2</sub>(s)+ Cl<sub>2</sub>(g)→ 2 ICl(g),Δ<sub>r</sub>H°
Q25: The enthalpy change for converting 10.0 g
Q26: Entropy is related to the way in
Q27: Standard molar entropy,S<sup>∘</sup>,increases as molecular complexity increases.
Q29: If the enthalpy of vaporization of chloromethane,CH<sub>3</sub>Cl,is
Q30: For Cl<sub>2</sub>O(g)+ 3/2 O<sub>2</sub>(g)→ 2 ClO<sub>2</sub> ,Δ<sub>r</sub>H°
Q31: The equilibrium constant for the reaction below
Q32: In the Haber process,ammonia is synthesized from
Q33: ΔG is positive for a spontaneous reaction.