Multiple Choice
Ethanol ( 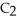
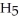 OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
A) 207.3 kJ
B) -12.7 kJ
C) 6.91 kJ
D) 4192 kJ
E) 9.21 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q2: What is Δ<sub>r</sub>H° for a reaction that
Q3: For CO(g)+ H<sub>2</sub>(g)→ H<sub>2</sub>CO(g),Δ<sub>r</sub>H° = -5.36 kJ/mol,and
Q4: For the reaction: CO(g)+ 2 H<sub>2</sub>(g)→ CH<sub>3</sub>OH(g)<br>K<sub>p</sub>
Q5: How much heat is released when 85.0
Q6: Which of the following substances under equal
Q7: Which of the listed reactions would couple
Q8: The equilibrium constant for the reaction below
Q9: The normal melting and boiling points of
Q10: The Gibbs energy change for a reaction
Q11: For the reaction PCl<sub>5</sub> (g)⇌ PCl<sub>3</sub> (g)+