Multiple Choice
In the Haber process,ammonia is synthesized from nitrogen and hydrogen: 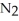 (g) +
(g) + 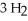 (g) →
(g) → 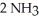 (g)
(g)
ΔrG ° at 298 K for this reaction is -33.3 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 1.9 atm 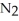 ,1.6 atm
,1.6 atm 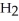 ,and 0.65 atm
,and 0.65 atm 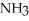 Is ________ kJ mol-1.
Is ________ kJ mol-1.
A) -1.8
B) -3.86 × 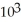
C) -7.25 × 103
D) -104.5
E) -40.5
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Standard molar entropy,S<sup>∘</sup>,increases as molecular complexity increases.
Q28: Choose the INCORRECT statement.<br>A)The van't Hoff equation
Q29: If the enthalpy of vaporization of chloromethane,CH<sub>3</sub>Cl,is
Q30: For Cl<sub>2</sub>O(g)+ 3/2 O<sub>2</sub>(g)→ 2 ClO<sub>2</sub> ,Δ<sub>r</sub>H°
Q31: The equilibrium constant for the reaction below
Q33: ΔG is positive for a spontaneous reaction.
Q34: An increase in entropy is observed when
Q35: If ΔG < 0 for a reaction,then
Q36: A spontaneous process:<br>A)will happen quickly<br>B)releases large amounts
Q37: The chemical potential of a substance,m,represents the