Multiple Choice
At 20 °C,an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 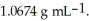 What is the molarity of ammonium chloride in the solution?
What is the molarity of ammonium chloride in the solution?
The formula weight of 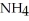 Cl is
Cl is 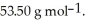
A) 5.90
B) 0.479
C) 4.79
D) 0.0445
E) 22.5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: An unknown compound is composed of 65.44%
Q32: What is the mass/vol % ethanol in
Q33: What is the correct value for the
Q34: What is the weight percent of vitamin
Q35: Calculate the freezing point of a solution
Q37: Liquid Q is a polar solvent and
Q38: A 1.00 molal solution of NaCl in
Q39: Which definition is INCORRECT?<br>A)mole fraction = moles
Q40: Commercial perchloric acid is 70.0% by mass,HClO<sub>4</sub>(aq).Calculate
Q41: A solution of LiCl in water has