Multiple Choice
Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water.The molal freezing point depression constant ( 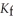 ) for water is
) for water is 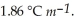
A) -0.45 °C
B) +0.45 °C
C) -0.23 °C
D) +0.23 °C
E) 1.23 °C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: 252 mL of 3.00 M H<sub>2</sub>SO<sub>4</sub>(aq)are added
Q31: An unknown compound is composed of 65.44%
Q32: What is the mass/vol % ethanol in
Q33: What is the correct value for the
Q34: What is the weight percent of vitamin
Q36: At 20 °C,an aqueous solution that is
Q37: Liquid Q is a polar solvent and
Q38: A 1.00 molal solution of NaCl in
Q39: Which definition is INCORRECT?<br>A)mole fraction = moles
Q40: Commercial perchloric acid is 70.0% by mass,HClO<sub>4</sub>(aq).Calculate