Multiple Choice
What is the hydroxide ion concentration and the pH for a hydrochloric acid solution that has a hydronium ion concentration of 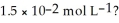
A) 6.7 × 10-12 mol L-1,2.82
B) 6.7 × 10-12 mol L-1,11.18
C) 6.7 × 10-13 mol L-1,1.82
D) 6.7 × 10-13 mol L-1,12.17
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: The ionization constant for ammonia is 1.8
Q20: The pH of a solution of NH<sub>4</sub>C<sub>2</sub>H<sub>3</sub>O<sub>2</sub>
Q21: What is the hydroxide ion concentration of
Q22: The concept of an acid not limited
Q23: Choose the Br∅nsted-Lowry acids and bases in
Q25: When comparing binary acids of the elements
Q26: What is the pH of a 0.570
Q27: List the following acids in order of
Q28: What is the pH of a 0.380
Q29: Calculate the pH of a 1.60 mol