Multiple Choice
Calculate the pH of a 1.60 mol L-1 aqueous KBrO solution.Ka for hypobromous acid,HBrO,is 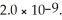
A) 2.55
B) 4.25
C) 9.75
D) 11.45
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: What is the hydroxide ion concentration and
Q25: When comparing binary acids of the elements
Q26: What is the pH of a 0.570
Q27: List the following acids in order of
Q28: What is the pH of a 0.380
Q30: What is the pH of a 0.040
Q31: What is the [H<sub>2</sub>AsO<sub>4</sub><sup>-</sup>] for an aqueous
Q32: In the equilibrium system described by: <br><img
Q33: In the reaction BF<sub>3</sub> + NH<sub>3</sub> →
Q34: What is the pH of a 0.361