Multiple Choice
What is the hydronium ion concentration and the pH for an aqueous solution of NH3 that has a hydroxide ion concentration of 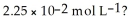
A) 4.44 × 10-12 mol L-1 and 2.648
B) 4.44 × 10-12 mol L-1 and 11.352
C) 4.44 × 10-13 mol L-1 and 1.648
D) 4.44 × 10-13 mol L-1 and 12.352
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q123: What is the hydronium ion concentration of
Q124: What is the pH of a 0.120
Q125: What is the [OH<sup>-</sup>] of a solution
Q126: Which of the following is the strongest
Q127: What is the pH of a 0.563
Q129: A saturated aqueous solution of calcium hydroxide
Q130: What is the [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] of an aqueous
Q131: What is the pH of a 0.475
Q132: Calculate the hydroxide ion concentration in an
Q133: What is the concentration of free sulfate