Multiple Choice
What is the hydronium ion concentration of a 0.150 mol L-1 aqueous hypochlorous acid solution with 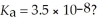
The equation for the dissociation of hypochlorous acid is below: 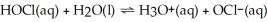
A) 1.9 × 10-4 mol L-1
B) 7.2 × 10-4 mol L-1
C) 2.8 × 10-5 mol L-1
D) 7.2 × 10-5 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q118: Calculate the pH of a 0.800 mol
Q119: A solution has pOH of -0.47.This means
Q120: According to the Arrhenius theory,a neutralization reaction
Q121: The K<sub>b</sub> value for methylamine is 4.2
Q122: In the reaction BF<sub>3</sub> + NH<sub>3</sub> ⇌
Q124: What is the pH of a 0.120
Q125: What is the [OH<sup>-</sup>] of a solution
Q126: Which of the following is the strongest
Q127: What is the pH of a 0.563
Q128: What is the hydronium ion concentration and