Multiple Choice
Calculate the molarity of hydroxide ion in an aqueous solution that has a pOH of 5.00.
A) 1.0 × 10-5
B) 9.00
C) 1.0 × 10-9
D) 5.0 × 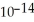
E) 9.0 × 10-14
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: What is the pH of a 0.500
Q38: What is the pH of a 0.052
Q39: A 0.505 g sample of a monoprotic
Q40: What is the indication of the relative
Q41: CO<sub>2</sub> acts as an acid in the
Q43: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q44: Calculate the pH for an aqueous solution
Q45: A 0.214 M aqueous solution of a
Q46: A 0.632 M aqueous solution of a
Q47: Choose the strongest acid.<br>A)HF<br>B)H<sub>2</sub>CO<sub>3 </sub><br>C)HCN<br>D)HC<sub>2</sub>H<sub>3</sub>O<sub>2 </sub><br>E)HClO<sub>4 </sub>