Multiple Choice
What is the pH of a 0.500 mol L-1 NH3 aqueous solution that has Kb = 1.8 × 10-5?
The equation for the dissociation of NH3 is below: 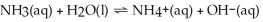
A) 2.22
B) 2.52
C) 11.48
D) 10.48
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: In the equilibrium system described by: <br><img
Q33: In the reaction BF<sub>3</sub> + NH<sub>3</sub> →
Q34: What is the pH of a 0.361
Q35: List the following acids in order of
Q36: According to the Arrhenius theory,a neutralization reaction
Q38: What is the pH of a 0.052
Q39: A 0.505 g sample of a monoprotic
Q40: What is the indication of the relative
Q41: CO<sub>2</sub> acts as an acid in the
Q42: Calculate the molarity of hydroxide ion in