Multiple Choice
An aqueous solution is prepared by dissolving 0.23 mol of hydrazoic acid and 0.27 mol of sodium azide in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly.The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution.The 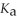
Of hydrazoic acid is 1.9 × 10-5.
A) 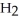 O
O
B) 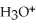
C) azide
D) hydrazoic acid
E) This is a buffer solution: the pH does not change upon addition of acid or base.
Correct Answer:

Verified
Correct Answer:
Verified
Q113: Phenol red indicator changes from yellow to
Q114: In a solution prepared by mixing equal
Q115: It is easier to calculate the pH
Q116: In the titration of a solution of
Q117: Calculate the pH of an aqueous solution
Q119: A 25.0 mL sample of 0.150 mol
Q120: Phenol red indicator changes from yellow to
Q121: Assuming no volume change on mixing,what mass
Q122: Mixing comparable amounts of a strong acid
Q123: What is the buffer range (for an