Multiple Choice
Calculate the pH of an aqueous solution that is 0.295 mol L-1 in sodium formate (HCOONa) and 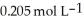 In formic acid (HCOOH) .The
In formic acid (HCOOH) .The 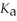 Of formic acid is 1.77 ×
Of formic acid is 1.77 × 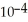 .
.
A) 3.910
B) 3.587
C) 13.84
D) 10.10
E) 4.963
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q112: Twenty-five milliliters of 0.10 M HCl(aq)is titrated
Q113: Phenol red indicator changes from yellow to
Q114: In a solution prepared by mixing equal
Q115: It is easier to calculate the pH
Q116: In the titration of a solution of
Q118: An aqueous solution is prepared by dissolving
Q119: A 25.0 mL sample of 0.150 mol
Q120: Phenol red indicator changes from yellow to
Q121: Assuming no volume change on mixing,what mass
Q122: Mixing comparable amounts of a strong acid