Multiple Choice
An aqueous solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 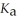
Of nitrous acid is 1.36 × 10-3.
A) 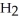 O
O
B) 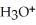
C) nitrite ion
D) nitrous acid
E) This is a buffer solution: the pH does not change at all upon addition of acid or base.
Correct Answer:

Verified
Correct Answer:
Verified
Q65: How will addition of sodium acetate to
Q66: Determine the pH of the following aqueous
Q67: The Henderson-Hasselbach equation,used to calculate the pH
Q68: Calculate the percent ionization of nitrous acid
Q69: Acid-base indicators have two forms: an acid
Q71: The buffer capacity of a solution may
Q72: Twenty-five milliliters of 0.10 M HCl(aq)is titrated
Q73: Which of the following can act as
Q74: What will the pH at the neutralization
Q75: A buffer was prepared by adding 2.4