Multiple Choice
Calculate the percent ionization of nitrous acid in an aqueous solution that is 0.266 mol L-1 in nitrous acid 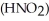 And 0.112 mol L-1 in potassium nitrite (
And 0.112 mol L-1 in potassium nitrite ( 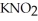 ) .The acid dissociation constant of nitrous acid is
) .The acid dissociation constant of nitrous acid is 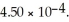
A) 29.6
B) 0.402
C) 11.2
D) 1.34 × 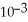
E) 1.55
Correct Answer:

Verified
Correct Answer:
Verified
Q63: Formic acid (HCOOH,K<sub>a</sub> = 1.8 × 10<sup>-4</sup>)is
Q64: For the following titration,determine whether the solution
Q65: How will addition of sodium acetate to
Q66: Determine the pH of the following aqueous
Q67: The Henderson-Hasselbach equation,used to calculate the pH
Q69: Acid-base indicators have two forms: an acid
Q70: An aqueous solution is prepared by dissolving
Q71: The buffer capacity of a solution may
Q72: Twenty-five milliliters of 0.10 M HCl(aq)is titrated
Q73: Which of the following can act as