Multiple Choice
A 25.0 mL sample of 0.150 mol L-1 aqueous hydrofluoric acid is titrated with a 0.150 mol L-1 NaOH(aq) solution.What is the pH at the equivalence point? The 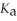
Of hydrofluoric acid is 3.5 × 10-4.
A) 10.17
B) 10.83
C) 3.17
D) 7.00
E) 8.17
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: An aqueous solution has [HC<sub>7</sub>H<sub>5</sub>O<sub>2</sub>] = 0.100
Q47: A 25.0 mL sample of 0.150 mol
Q48: What is the pH of a buffer
Q49: Determine the pH of the following aqueous
Q50: Determine the pH of the following aqueous
Q52: A handbook states that to prepare a
Q53: Choose the expression that gives the molar
Q54: For an accurate titration,the end point needs
Q55: What is the change in pH after
Q56: A pH 9.56 buffer was prepared by