Multiple Choice
A 25.0 mL sample of 0.150 mol L-1 aqueous hypochlorous acid is titrated with a 0.150 mol L-1 NaOH(aq) solution.What is the pH after 26.0 mL of base is added? The 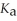
Of hypochlorous acid is 3.0 × 10-8.
A) 2.54
B) 11.47
C) 7.00
D) 7.51
E) 7.54
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: What is the approximate pH at the
Q43: What is the pH of a solution
Q44: What volume of 5.00 × 10<sup>-3</sup> mol
Q45: A salt of a polyprotic acid such
Q46: An aqueous solution has [HC<sub>7</sub>H<sub>5</sub>O<sub>2</sub>] = 0.100
Q48: What is the pH of a buffer
Q49: Determine the pH of the following aqueous
Q50: Determine the pH of the following aqueous
Q51: A 25.0 mL sample of 0.150 mol
Q52: A handbook states that to prepare a