Multiple Choice
What is the pH of a solution made by mixing 10.00 mL of 0.10 mol L-1 aqueous acetic acid with 10.00 mL of 0.10 mol L-1 KOH(aq) ? Assume that the volumes of the solutions are additive.Ka = 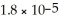
For CH3COOH.
A) 5.28
B) 7.00
C) 8.72
D) 10.02
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Which of the following mixtures would you
Q32: What is the pH of an aqueous
Q33: Determine the pH of the following aqueous
Q34: What is the concentration of the acetate
Q35: Phenol red indicator changes from yellow to
Q37: The color change range of most acid-base
Q38: Phenol red indicator changes from yellow to
Q39: For the following titration,determine whether the solution
Q40: Choose the correct statement.<br>A)30 mL of 2
Q41: A pH 4.88 buffer was prepared by