Multiple Choice
What is the molar solubility of AgCl in 0.40 mol L-1 NH3(aq) ? Ksp for AgCl(s) is 1.8 × 10-10 and Kf for Ag(NH3) 2+ is 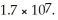
A) 1.3 × 10-5 mol L-1
B) 2.0 × 10-5 mol L-1
C) 2.2 × 10-2 mol L-1
D) 5.5 × 10-2 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q11: An aqueous solution contains [Ba<sup>2+</sup>] = 5.0
Q12: Concentrated aqueous solutions of iron (III)chloride,sodium hydroxide,and
Q13: What is the molar solubility of barium
Q14: When 100 mL each of 2.0 ×
Q15: Qualitative cation analysis has been replaced in
Q17: The solubility product constant of Li<sub>3</sub>PO<sub>4</sub> is
Q18: The common ion effect is a case
Q19: Consider an aqueous solution which is 0.020
Q20: Calculate the K<sub>sp</sub> for silver carbonate if
Q21: The solubility product constant of PbI<sub>2</sub>(s)is 7.1