Multiple Choice
Solid potassium chromate (K2CrO4) is slowly added to a solution containing 0.50 mol L-1 AgNO3(aq) and 0.50 mol L-1 Ba(NO3) 2(aq) .What is the Ag+(aq) concentration when BaCrO4(s) just starts to precipitate?
The Ksp for Ag2CrO4(s) and BaCrO4(s) are 1.1 × 10-12 and 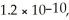 Respectively.
Respectively.
A) 6.5 × 10-5 mol L-1
B) 1.3 × 10-4 mol L-1
C) 3.2 × 10-4 mol L-1
D) 6.8 × 10-2 mol L-1
Correct Answer:

Verified
Correct Answer:
Verified
Q78: Consider an aqueous solution which is 0.020
Q79: What is the free Ag<sup>+</sup>(aq)concentration of 0.020
Q80: A saturated solution of silver chromate has
Q81: Concentrated aqueous solutions of sodium sulfate,ammonium carbonate,and
Q82: What is the minimum pH at which
Q84: In a qualitative cation analysis,the unknown ion
Q85: In a qualitative cation analysis,the unknown ion
Q86: The solubility of a salt MX<sub>2</sub> with
Q87: A solution of NaF(aq)is added dropwise to
Q88: Write the solubility product constant expression for