Multiple Choice
A solution of NaF(aq) is added dropwise to a solution that is 0.0102 mol L-1 in 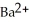 (aq) .When the concentration of
(aq) .When the concentration of 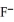 (aq) exceeds ________ mol L-1,
(aq) exceeds ________ mol L-1, 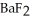 (s) will precipitate.Neglect volume changes.For
(s) will precipitate.Neglect volume changes.For 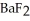
(s) , 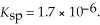
A) 8.6 × 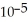
B) 1.3 × 10-2
C) 1.7 × 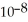
D) 3.3 × 
E) 1.7 × 10-4
Correct Answer:

Verified
Correct Answer:
Verified
Q82: What is the minimum pH at which
Q83: Solid potassium chromate (K<sub>2</sub>CrO<sub>4</sub>)is slowly added to
Q84: In a qualitative cation analysis,the unknown ion
Q85: In a qualitative cation analysis,the unknown ion
Q86: The solubility of a salt MX<sub>2</sub> with
Q88: Write the solubility product constant expression for
Q89: When 100 mL each of 2.0 ×
Q90: Which of the following salts,each of which
Q91: An aqueous solution contains [Ba<sup>2+</sup>] = 5.0
Q92: If chromium(III)chloride is added to be 1