Multiple Choice
The standard Gibbs energy change for the following voltaic cell is ΔG° = -89.3 kJ at 25 °C.What is E°cell? 
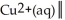
 Ag(s)
Ag(s)
A) +0.463 V
B) -0.926 V
C) -0.463 V
D) +0.926 V
E) +0.231 V
Correct Answer:

Verified
Correct Answer:
Verified
Q87: Determine E°<sub>cell</sub> for the reaction: Pb<sup>2+</sup>(aq)+ Zn(s)→
Q88: Calculate E<sub>cell </sub>at 25 °C for the
Q89: What is the shorthand notation that represents
Q90: For the following voltaic cell,determine the [Cl<sup>-</sup>]
Q91: A solution of Al(NO<sub>3</sub>)<sub>3</sub>(aq)is electrolyzed by passing
Q93: Determine E°<sub>cell</sub> for the reaction: 2 Al(aq)+
Q94: Determine E°<sub>cell</sub> for the reaction: MnO<sub>4</sub><sup>-</sup>(aq)+ 8
Q95: In a galvanic cell,oxidation occurs at the
Q96: Find E<sub>cell</sub> at 25 °C for the
Q97: Determine E°<sub>cell</sub> for the reaction: 2 Al<sup>3+</sup>(aq)+