Multiple Choice
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten 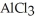 (l) with an electrical current of 12.0 A?
(l) with an electrical current of 12.0 A?
A) 27.0
B) 9.00
C) 1.19 × 103
D) 2.90 × 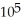
E) 3.57 × 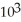
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Using the following reduction half-reactions and their
Q3: Determine E°<sub>cell</sub> for the reaction: I<sub>2</sub>(s)+ Cu(s)→
Q4: What is the reduction half-reaction for the
Q5: When E<sub>cell</sub> < 0,the reaction is spontaneous.
Q6: How long must a constant current of
Q7: What is E° of the spontaneous cell
Q8: Write the net redox reaction that occurs
Q9: What is the cell reaction of the
Q10: 3.53 L of 0.362 M CuCl<sub>2</sub>(aq)solution are
Q11: E°<sub>cell</sub> is the standard cell potential.