Multiple Choice
Which of the following represents a disproportionation reaction?
A) 2 Cu+(aq) + H2O(l) → Cu(s) + CuO(s) + 2 H+(aq)
B) 2 CrO42-(aq) + 2 H+(aq) → Cr2O72-(aq) + H2O(aq)
C) TiCl4(l) + 2 H2O(l) → TiO2(s) + 4 HCl(g)
D) MnO4-(aq) + 5 VO2+(aq) + H2O(l) → Mn2+(aq) + 5 VO2+(aq) + 2 H+(aq)
E) 3MnO2(s) + 6 KOH(aq) + KClO3(aq) 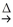 3 K2MnO4(aq) + KCl(aq) + 3 H2O(l)
3 K2MnO4(aq) + KCl(aq) + 3 H2O(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q98: In the group of elements Cr,Ni,Sn,Pt,Ti,the one
Q99: The high melting points of transition elements
Q100: What is the ground-state electron configuration for
Q101: Choose the INCORRECT statement about the electron
Q102: Choose the INCORRECT statement about metallurgy.<br>A)Extractive metallurgy
Q103: Choose the INCORRECT statement.<br>A)Cu,Ag,and Au are the
Q104: Choose the INCORRECT statement about transition elements.<br>A)Ferromagnetism
Q105: List the following in order of increasing
Q106: If an aqueous solution is prepared by
Q107: Write the electron configuration for Co<sup>3+</sup>.<br>A)[Ar]3d<sup>6 </sup><br>B)[Ar]4s<sup>2</sup>3d<sup>4