Multiple Choice
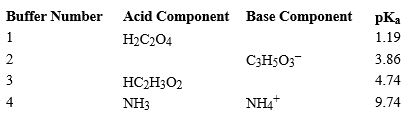 Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...) indicating the buffer and/or the letter indicating the formula below.
Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...) indicating the buffer and/or the letter indicating the formula below.
-In Buffer 2,_________________________ would react with added acid.
A) H3C2O4+
B) HC2O4-
C) C3H4O32-
D) HC3H5O3
E) C2H3O2-
F) H2C2H3O2
G) C3H5O3-
Correct Answer:

Verified
Correct Answer:
Verified
Q72: Consider the following three buffer reactions.<br>Buffer 1:
Q73: Below is the structure for the amino
Q74: If you dissolve 0.10 mol of a
Q75: When fumaric acid, H<sub>2</sub>C<sub>4</sub>H<sub>2</sub>O<sub>4</sub> reacts with NaOH,
Q76: An ammonia solution has a pH of
Q77: The following reaction represents the self-ionization of
Q78: The pK<sub>a</sub> of lactic acid is 3.86,
Q80: Which of the following is a polyprotic
Q81: A buffer is composed of ammonia (NH<sub>3</sub>)
Q82: Human blood contains which of the following