Short Answer
The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq) 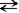 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In the reverse reaction, __________________________ion functions as an acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q72: Consider the following three buffer reactions.<br>Buffer 1:
Q73: Below is the structure for the amino
Q74: If you dissolve 0.10 mol of a
Q75: When fumaric acid, H<sub>2</sub>C<sub>4</sub>H<sub>2</sub>O<sub>4</sub> reacts with NaOH,
Q76: An ammonia solution has a pH of
Q78: The pK<sub>a</sub> of lactic acid is 3.86,
Q79: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1423/.jpg" alt=" Using the above
Q80: Which of the following is a polyprotic
Q81: A buffer is composed of ammonia (NH<sub>3</sub>)
Q82: Human blood contains which of the following