Essay
The structure of urea is shown below.Fill in any nonbonding valence electrons that are missing from the line-bond structure.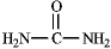
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: In the two structures shown below,what do
Q8: How many nonbonding electron pairs are in
Q9: The following species forms during an organic
Q11: Draw a picture showing the orbitals involved
Q15: The molecular formula C<sub>2</sub>H<sub>4</sub>O can be converted
Q20: Draw two possible isomers of C<sub>6</sub>H<sub>6</sub> in
Q27: In drawing the Lewis structure for an
Q28: According to atomic theory:<br>A) the nucleus is
Q32: Instructions: Consider the two structures below
Q34: How many total valence electrons are represented