Multiple Choice
The molecular formula C2H4O can be converted into three-line bond (Kekulé) structures that are consistent with valence rules.Which one of the following Kekulé structures is not consistent with valence rules?
A) 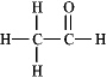
B) 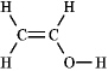
C) 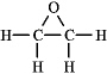
D) 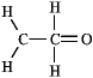
Correct Answer:

Verified
Correct Answer:
Verified
Q10: The structure of urea is shown below.Fill
Q10: Consider the formation of an sp<sup>2</sup> hybrid
Q11: Draw a picture showing the orbitals involved
Q19: How many electrons are there in the
Q25: Consider the two structures below to answer
Q26: Instructions: Propose a structure for a molecule
Q27: In drawing the Lewis structure for an
Q32: Instructions: Consider the two structures below
Q34: How many total valence electrons are represented
Q38: Draw the structure for CCl<sub>2</sub>F<sub>2</sub> using solid,