Multiple Choice
Calculate the standard enthalpy change for the reaction
2C8H18(l) + 17O2(g) 16CO(g) + 18H2O(l) .
Given: 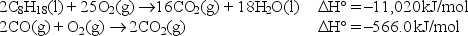
A) -10.450 kJ/mol
B) -6,492 kJ/mol
C) 6,492 kJ/mol
D) 10,450 kJ/mol
E) 15,550 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: The heat of solution of KCl is
Q109: Calculate the amount of work done, in
Q110: If 10.6 moles of water at 35°C
Q111: The enthalpy of combustion of acetylene
Q112: The enthalpy change when a strong acid
Q113: When an automobile engine starts, the metal
Q114: Solid sodium peroxide (Na<sub>2</sub>O<sub>2</sub>)reacts with liquid
Q116: Pentaborane B<sub>5</sub>H<sub>9</sub>(s)burns vigorously in O<sub>2</sub> to
Q117: The heat absorbed by a system
Q118: The specific heats of water and iron