Multiple Choice
During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation:
2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g)
Calculate the standard enthalpy change for the above reaction given: 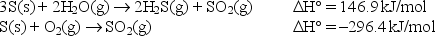
A) -1036.1 kJ/mol
B) -742.3 kJ/mol
C) -149.5 kJ/mol
D) 443.3 kJ/mol
E) 742.3 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Which of the following processes is
Q51: A piece of copper with a mass
Q52: Aluminum oxide can be reduced to
Q53: Calculate the standard enthalpy change for
Q54: An exothermic reaction causes the surroundings to<br>A)increase
Q56: Calculate the standard enthalpy change for
Q57: Given that CaO(s)+ H<sub>2</sub>O(l) <span class="ql-formula"
Q58: Concerning the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="Concerning the
Q59: What would be the standard enthalpy
Q60: Naphthalene combustion can be used to calibrate