Multiple Choice
The isomerization of cyclopropane to form propene is a first-order reaction. 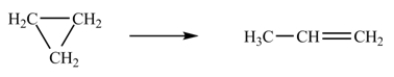 At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min.Determine the rate constant for this reaction at 760 K.
At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min.Determine the rate constant for this reaction at 760 K.
A) 3.66 × 10-2 min-1
B) 1.04 × 10-2 min-1
C) 2.42 min-1
D) 2.06 × 10-3 min-1
E) 2.40 × 10-2 min-1
Correct Answer:

Verified
Correct Answer:
Verified
Q1: At a particular temperature the first-order
Q4: Appropriate units for a second-order rate constant
Q7: The activation energy for the reaction
Q11: In general, to calculate the time required
Q31: In general, to calculate the rate
Q83: For the first-order reaction 2N<sub>2</sub>O<sub>5</sub>
Q85: Given the rate law for a reaction,
Q88: For the chemical reaction system described by
Q91: A certain reaction A <span
Q120: For the chemical reaction A