Multiple Choice
For the chemical reaction system described by the diagram below, which statement is true? 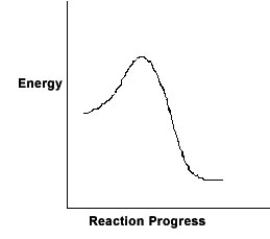
A) The forward reaction is endothermic.
B) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction.
C) At equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction.
D) The activation energy for the reverse reaction is greater than the activation energy for the forward reaction.
E) The reverse reaction is exothermic.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Appropriate units for a second-order rate constant
Q7: The activation energy for the reaction
Q11: In general, to calculate the time required
Q31: In general, to calculate the rate
Q83: For the first-order reaction 2N<sub>2</sub>O<sub>5</sub>
Q85: Given the rate law for a reaction,
Q87: The isomerization of cyclopropane to form propene
Q91: A certain reaction A <span
Q93: Given the rate law for a reaction,
Q120: For the chemical reaction A