Multiple Choice
Calculate the activation energy, in kJ/mol, for the redox reaction
Sn2+ + 2Co3+ Sn4+ + 2Co2+. 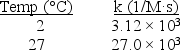
A) 59.2
B) 0.477
C) 5.37
D) 163 kJ
E) 48.1 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The half life for a first order
Q9: Concerning the rate law, Rate = k[A][B][C],
Q64: In general, to calculate the rate constant
Q96: An experimental drug, D, is known to
Q97: A reaction is experimentally found to follow
Q99: At 25°C, the second-order reaction NOCl(g)
Q101: The data below were determined for
Q103: The isomerization of cyclopropane to propene follows
Q104: The following reaction in aqueous solution
Q112: The Arrhenius equation is k = Ae<sup>-Ea/RT</sup>.