Multiple Choice
The isomerization of cyclopropane to propene follows first-order kinetics. 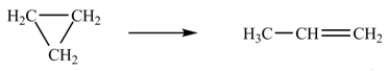 At 700 K, the rate constant for this reaction is 6.2 × 10-4 min-1.How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
At 700 K, the rate constant for this reaction is 6.2 × 10-4 min-1.How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
A) 16,100 min
B) 170 min
C) 3,710 min
D) 1.43 × 10-3 min
E) 1,120 min
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Concerning the rate law, Rate = k[A][B][C],
Q62: For a second order reaction, the half-life
Q64: In general, to calculate the rate constant
Q99: At 25°C, the second-order reaction NOCl(g)
Q100: Calculate the activation energy, in kJ/mol,
Q101: The data below were determined for
Q104: The following reaction in aqueous solution
Q107: Use the table of data shown
Q108: The first-order decomposition of SO<sub>2</sub>Cl<sub>2</sub> to sulfur
Q112: The Arrhenius equation is k = Ae<sup>-Ea/RT</sup>.