Short Answer
Use the table of data shown below to calculate the average rate of the reaction A B between 10 s and 20 s. 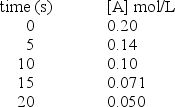
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Which one of the following changes
Q62: For a second order reaction, the half-life
Q64: In general, to calculate the rate constant
Q70: Which of the following elementary steps
Q103: The isomerization of cyclopropane to propene follows
Q104: The following reaction in aqueous solution
Q108: The first-order decomposition of SO<sub>2</sub>Cl<sub>2</sub> to sulfur
Q112: The Arrhenius equation is k = Ae<sup>-Ea/RT</sup>.
Q112: The first-order decomposition of phosphene to
Q121: For the hypothetical reaction A +