Multiple Choice
In the reaction HSO4-(aq) + OH-(aq) 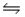 SO42-(aq) + H2O(l) , the conjugate acid-base pairs are
SO42-(aq) + H2O(l) , the conjugate acid-base pairs are 
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: If the pH of liquid bleach is
Q4: Which of the following yields a basic
Q6: The equilibrium constant for the reaction C<sub>7</sub>H<sub>15</sub>COOH(aq)+
Q7: The pH of a 0.6 M solution
Q8: Which of these acids is stronger, H<sub>2</sub>SO<sub>4</sub>
Q9: Determine the pH of a KOH solution
Q10: Which of the following solutions is acidic?<br>A)[H<sub>3</sub>O<sup>+</sup>]
Q11: The pOH of a solution is 10.40.Calculate
Q68: What is the pH of a 0.014
Q162: Which of these species will act as