Multiple Choice
The equilibrium constant for the reaction C7H15COOH(aq) + HCOO-(aq) 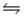
C7H15COO-(aq) + HCOOH(aq)
Is 7.23 × 10-2 at 25°C.If Ka for formic acid (HCOOH) is 1.77 × 10-4, what is the acid dissociation constant for C7H15COOH?
A) 2.45 × 10-3
B) 4.08 × 10-2
C) 7.81 × 10-4
D) 1.00 × 10-4
E) 1.28 × 10-5
Correct Answer:

Verified
Correct Answer:
Verified
Q3: If the pH of liquid bleach is
Q4: Which of the following yields a basic
Q5: In the reaction HSO<sub>4</sub><sup>-</sup>(aq)+ OH<sup>-</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q7: The pH of a 0.6 M solution
Q8: Which of these acids is stronger, H<sub>2</sub>SO<sub>4</sub>
Q9: Determine the pH of a KOH solution
Q10: Which of the following solutions is acidic?<br>A)[H<sub>3</sub>O<sup>+</sup>]
Q11: The pOH of a solution is 10.40.Calculate
Q68: What is the pH of a 0.014
Q162: Which of these species will act as