Multiple Choice
A 0.10 M NH3 solution is 1.3% ionized.Calculate the H+ ion concentration. NH3 + H2O 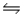
NH4+ + OH-
A) 1.3 × 10-3 M
B) 7.7 × 10-2 M
C) 7.7 × 10-12 M
D) 0.13 M
E) 0.10 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: Which one of the following equations represents
Q40: Calculate the hydrogen ion concentration in a
Q44: Calculate the H<sup>+</sup> ion concentration in a
Q45: Consider the weak bases below and their
Q46: Consider the weak acid CH<sub>3</sub>COOH (acetic acid).If
Q47: Identify the conjugate acid-base pairs in the
Q61: Identify the conjugate base of HCO<sub>3</sub><sup>-</sup>.<br>A) H<sub>2</sub>CO<sub>3</sub><br>B)
Q89: What is the concentration of H<sup>+</sup> in
Q146: A 5.2 L sample of a 1.1
Q153: Calculate the pH of a 0.10 M