Essay
Identify the conjugate acid-base pairs in the reaction HSO4- + HF 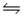
H2SO4 + F-
One conjugate acid-base pair is _______________; the other acid-base pair is ______________.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: A 0.10 M NH<sub>3</sub> solution is 1.3%
Q44: Calculate the H<sup>+</sup> ion concentration in a
Q45: Consider the weak bases below and their
Q46: Consider the weak acid CH<sub>3</sub>COOH (acetic acid).If
Q49: The pOH of a solution is 9.60.Calculate
Q50: What is the OH<sup>-</sup> ion concentration in
Q67: Which of the following does not fit
Q89: What is the concentration of H<sup>+</sup> in
Q146: A 5.2 L sample of a 1.1
Q155: Calculate the pH of a 0.14 M