Multiple Choice
For the reaction 2 SO2(g) + O2(g) 2 SO3(g) , if initially P(SO2) = 1.2 atm, P(O2) = 1.8 atm, and P(SO3) = 2.1 atm, calculate G for this reaction at 25°C.The following data is valid at 25°C: 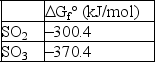
A) -140.0 kJ/mol
B) -137.6 kJ/mol
C) -138.7 kJ/mol
D) 1,174.7 kJ/mol
E) -141.3 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Arrange these reactions according to increasing
Q86: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q87: Which of the following is consistent
Q88: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q89: Calculate the equilibrium constant for the
Q90: The standard free energy of formation of
Q92: In the gas phase, formic acid
Q94: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q95: Which of these species would you expect
Q96: Sodium carbonate can be made by