Short Answer
For the reaction SbCl5(g) 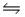
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate G at 800 K and 1 atm pressure (assume S° and H° do not change with temperature).
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Arrange these reactions according to increasing
Q89: Calculate the equilibrium constant for the
Q90: The standard free energy of formation of
Q91: For the reaction 2 SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q92: In the gas phase, formic acid
Q95: Which of these species would you expect
Q96: Sodium carbonate can be made by
Q97: Given the following data, calculate the boiling
Q98: For the reaction H<sub>2</sub>O<sub>2</sub>(g) <span class="ql-formula"
Q99: Predict the normal boiling point of triethylborane