Multiple Choice
In the gas phase, methyl isocyanate (CH3NC) isomerizes to acetonitrile (CH3CN) , H3C-N C (g) 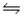
H3C-C N (g)
With H° = -89.5 kJ/mol and G° = - 73.8 kJ/mol at 25°C.Find the equilibrium constant for this reaction at 100°C.
A) 1.68 × 10-10
B) 5.96 × 109
C) 2.16 × 1010
D) 4.63 × 10-11
E) 8.64 × 1012
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Using the thermodynamic data provided below, calculate
Q42: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q43: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img
Q45: Calculate the free energy of formation
Q47: For any pure substance, if
Q48: What is the free energy change
Q49: Which response includes all of the following
Q50: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q51: Using the thermodynamic data provided below, calculate
Q126: The standard entropy of any pure substance